Our publications
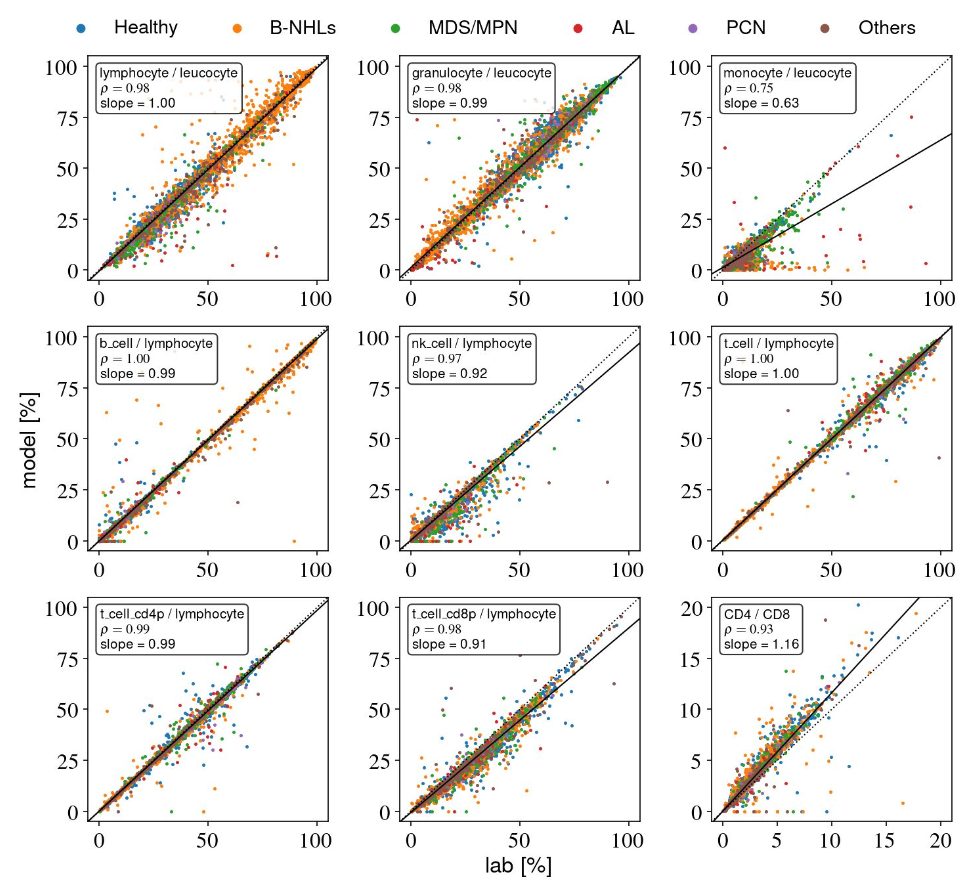
Leukocyte differentiation study
We're very proud of this strong work on AI cytometry led by our partners from the HpH Institut für Pathologie und Hämatopathologie Hamburg and Pathodiagnostik Berlin Mvz GmbH! This major retrospective comparison across >6,500 peripheral-blood samples shows how far AI-based cytometry has come: very high concordance with conventional lab results across major subpopulations, robustness across multiple panels, workflows and laboratory locations, and a clear view of where harmonization is still needed. Exactly the kind of evidence that matters for real-world laboratory cytometry. You can find the full poster here.

hema.to speeds up diagnostic workflow and improves quality
We're incredibly proud to share that hema.to has been clinically validated in an international, four-center clinical trial! Blood cancer diagnoses weren't just twice as fast, they were made with 8% more sensitivity and 10% more specificity when made with the help of our beautiful, easy-to-use decision-support software. You can download the white paper here.

Knowledge transfer enhances performance
... as shown by Nanditha Mallesh and co-authors in this Cell Patterns paper. In this collaboration between the hema.to team, the Munich Leukemia Lab, Charité and additional renowned university clinics, we showed that data from multiple labs can be combined to increase the clinical performance of AI models for each lab.

Deep learning can classify blood cancer on an expert level
... as shown by the hema.to team in collaboration with several experienced hematologists and the university of Bonn in this Cytometry A paper. With this paper, Zhao et al. have shown deep learning can be used to aid the routine clinical workflow using cytometry data.

Knowledge transfer enhances performance
... as shown by Nanditha Mallesh and co-authors in this 2019 Blood paper. With this paper, we showed that improvement of diagnostic performance is generated by combining multiple datasets, an approach we call "knowledge transfer".

Neural nets provide highly reliable diagnostic support in a routine setting
... as shown by a collaboration between hema.to, the Munich Leukemia Lab and the University of Bonn in this 2019 Blood paper. This paper shows that AI can support hematologists in their routine workflows for blood cancer diagnosis by providing highly reliable recommendations.
Frequently Asked Questions
We offer training and support free-of-charge.
Besides email support users can schedule a meeting with us to ensure they receive the assistance they need from the most suitable expert.
Yes, you can adjust the AI's suggestions with a few clicks. We have e.g. developed an easy-to-use tool which you can use to manually relabel cell types.
You can keep using your current cytometer / panel / workflow without changing anything. This feature together with hema.to's powerful analysis capabilities is unique in the market.
hema.to CellStudio (RUO) requires ~20 fcs/lmd files per panel to train and validate expert-level AI models.
hema.to BNHL product (CE-IVD) additionally provides diagnostic recommendations and therefore requires more files to be trained properly.








